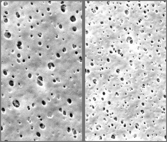
 DPPS Filters are bacteria retention filters consisting of two layers of Polyethersulfone (PES) membrane. The bioburden reduction prefilter and the sterilizing grade final filter each come in several pore sizes to meet unique requirements for your application.
DPPS Filters are bacteria retention filters consisting of two layers of Polyethersulfone (PES) membrane. The bioburden reduction prefilter and the sterilizing grade final filter each come in several pore sizes to meet unique requirements for your application.
DPPS filters are 100% integrity tested. They are developed and manufactured under an ISO 9001:2015 certified Quality Management System and designed to be used in cGMP-compliant processes.
DPPS filters are validated using test procedures that comply with ASTM F 838-15(ae1) protocols for the determination of bacterial retention in filters used for liquid filtration. The challenge level is a minimum of 107 organisms per cm2 of filter media. CPF filters have > 7-log removal when challenged with the organisms listed below (0.03μm, 0.10μm, 0.10Mμm, and 0.22μm meet the FDA definition of sterilizing grade filters). Validation Guides are available upon request.

It's simple. Our products are very competitively priced. Learn more

.png?width=100&name=90012015Large-(1).png)